Market, PMS and Vigilance Solutions
MedBoard Platform,
it is all it takes to transform your Market Intelligence and PMS
Global Market and Vigilance Databases integrated with Software solutions.
Professional solutions for each step of an effective
Market Research, Intelligence and Vigilance
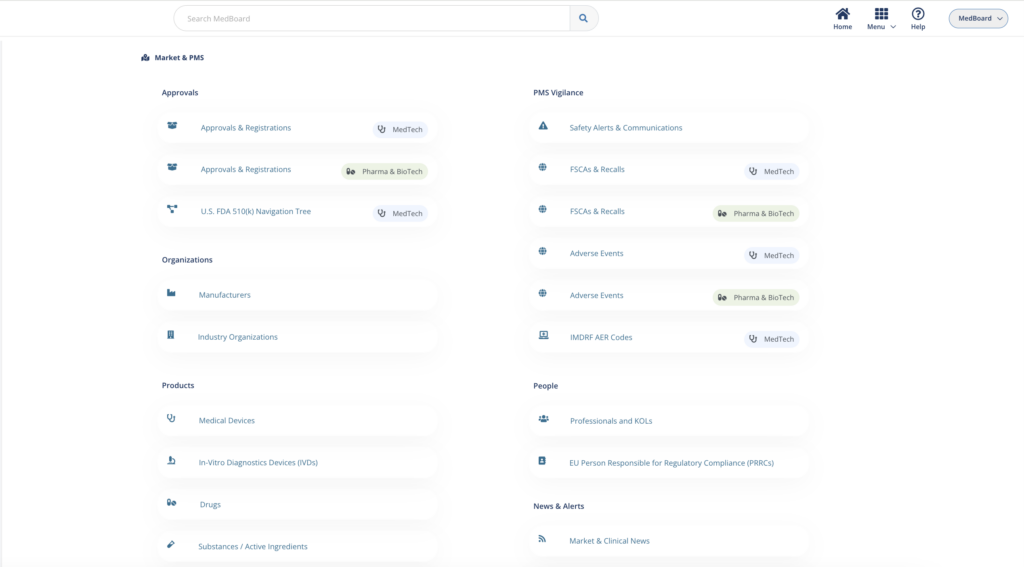
Global market databases
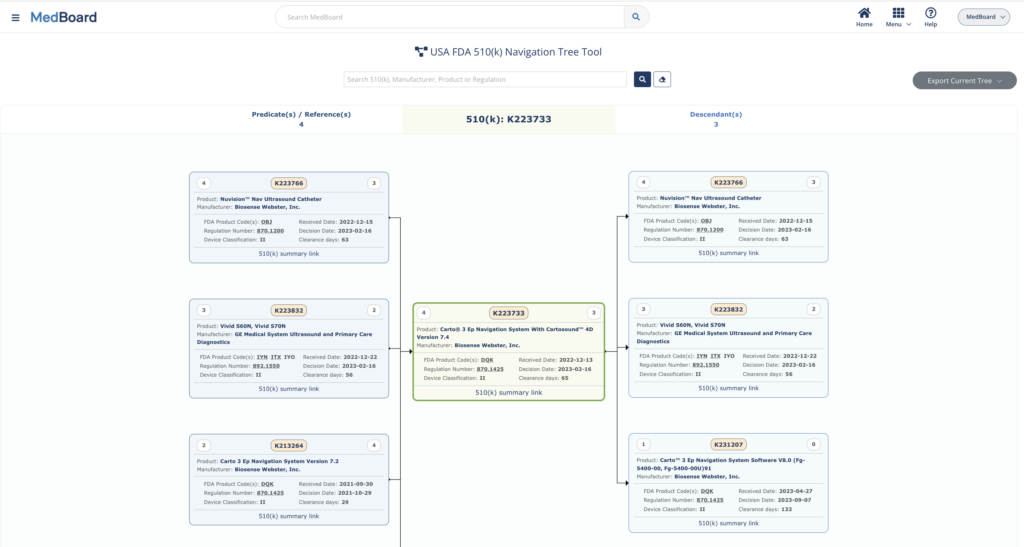
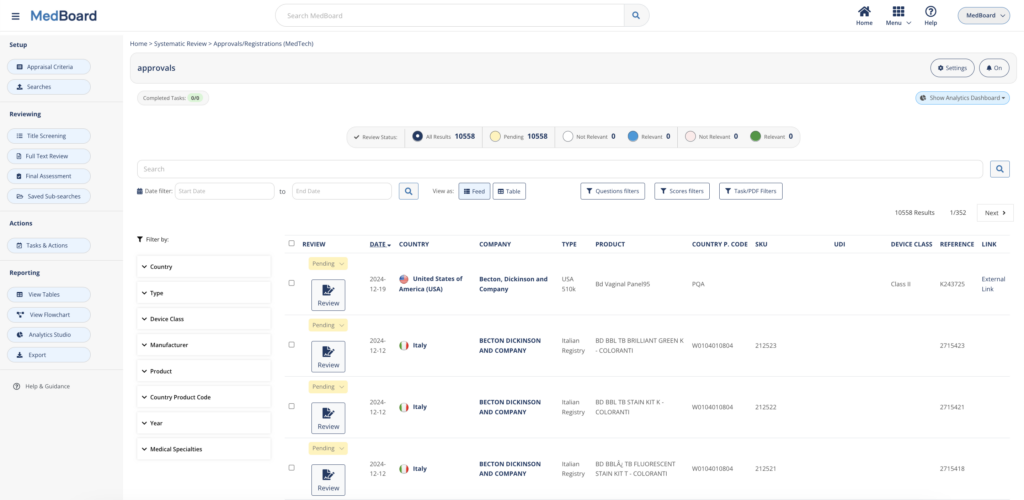
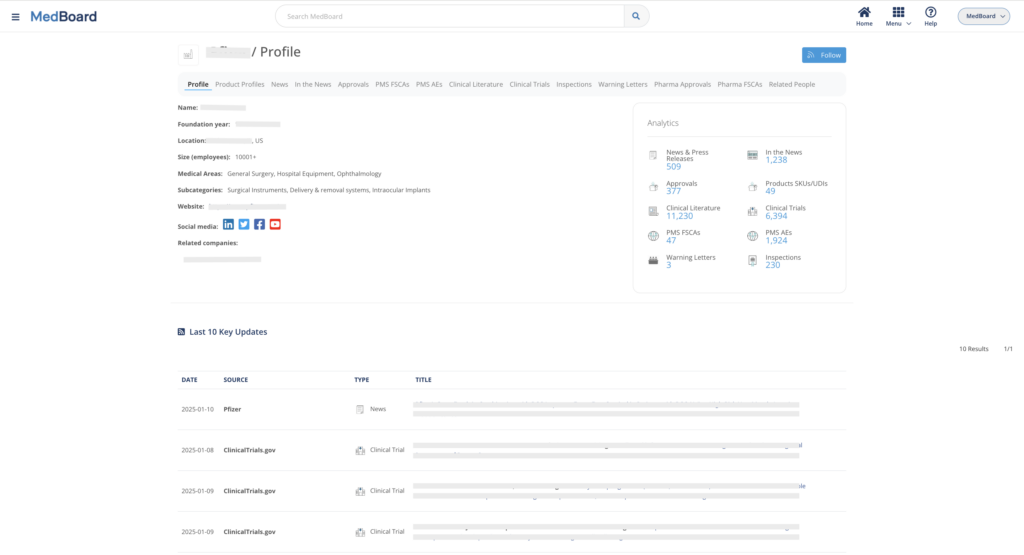
Access to up to date Market databases including millions of international Approvals & Registrations, USA 510(k) Navigation Tree tool, Medical Products profiles (Devices, IVDs, Drugs, Substances, Apps, etc.), Medical Manufacturers, Professionals and KOLs, market news and much more.
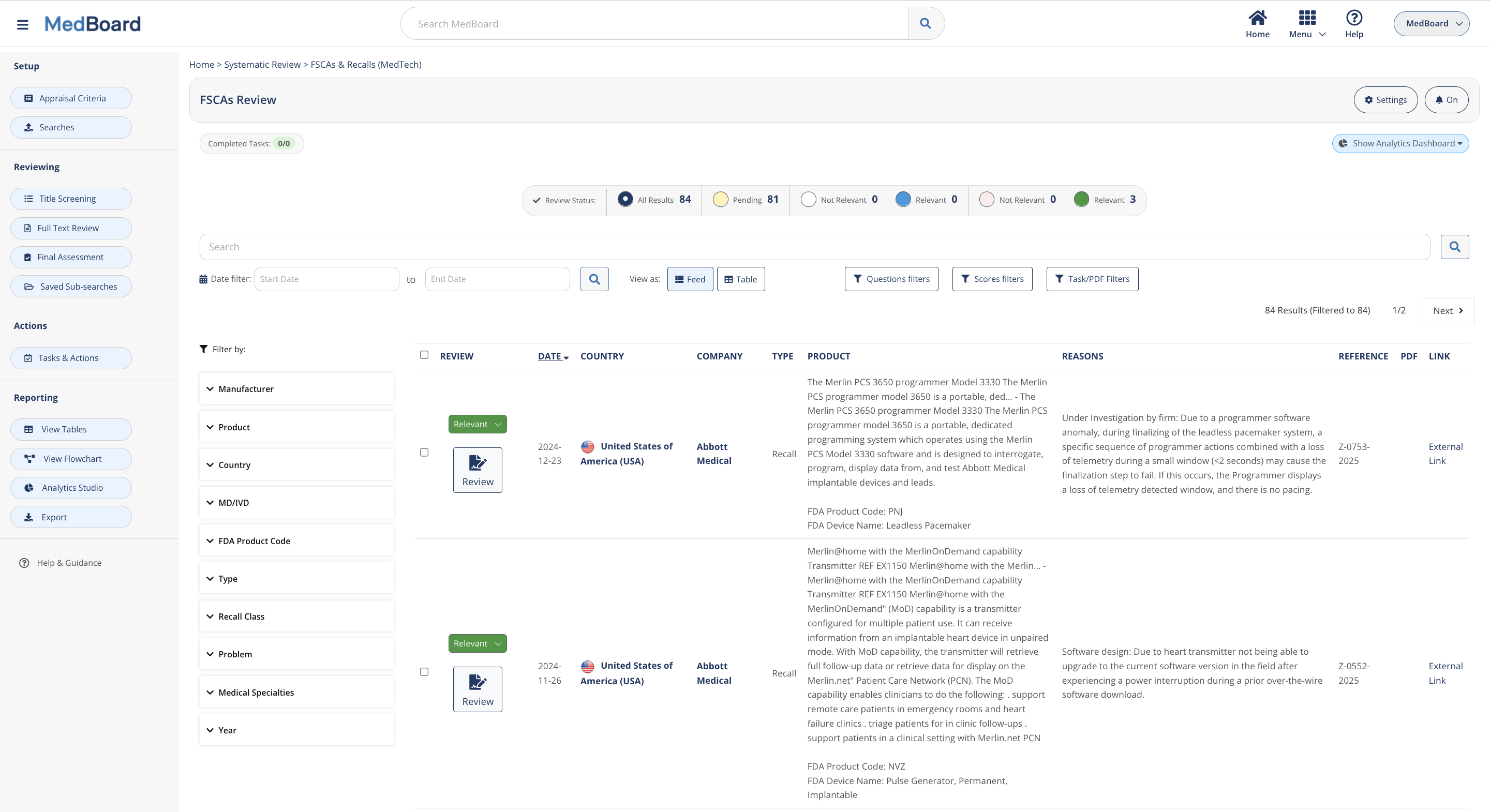
Vigilance Databases include Safety Alerts, FSCAs & Recalls, Adverse Events and Shortages. Integrated with advanced filters, analytics and integrations with other MedBoard databases
Monitoring and systematic review tools
Perform systematic monitoring and reviews of market databases such as Approvals and Registrations, press releases, media news, and PMS Vigilance databases such as PMS Adverse Events, Recalls, FSCAs, Safety Alerts. Customize the queries and perform proactive Monitoring, Impact Assessment, and Reporting in a systematic, easy and integrated process. Features include:
- Real time updates and notifications
- Appraisal process for a consistent review framework to assess impact
- AI support for data collection and extraction
- A collaborative process for seamless execution by the team
- Ready to use reporting tools
Continuous monitoring, implementation and reporting.Monitor
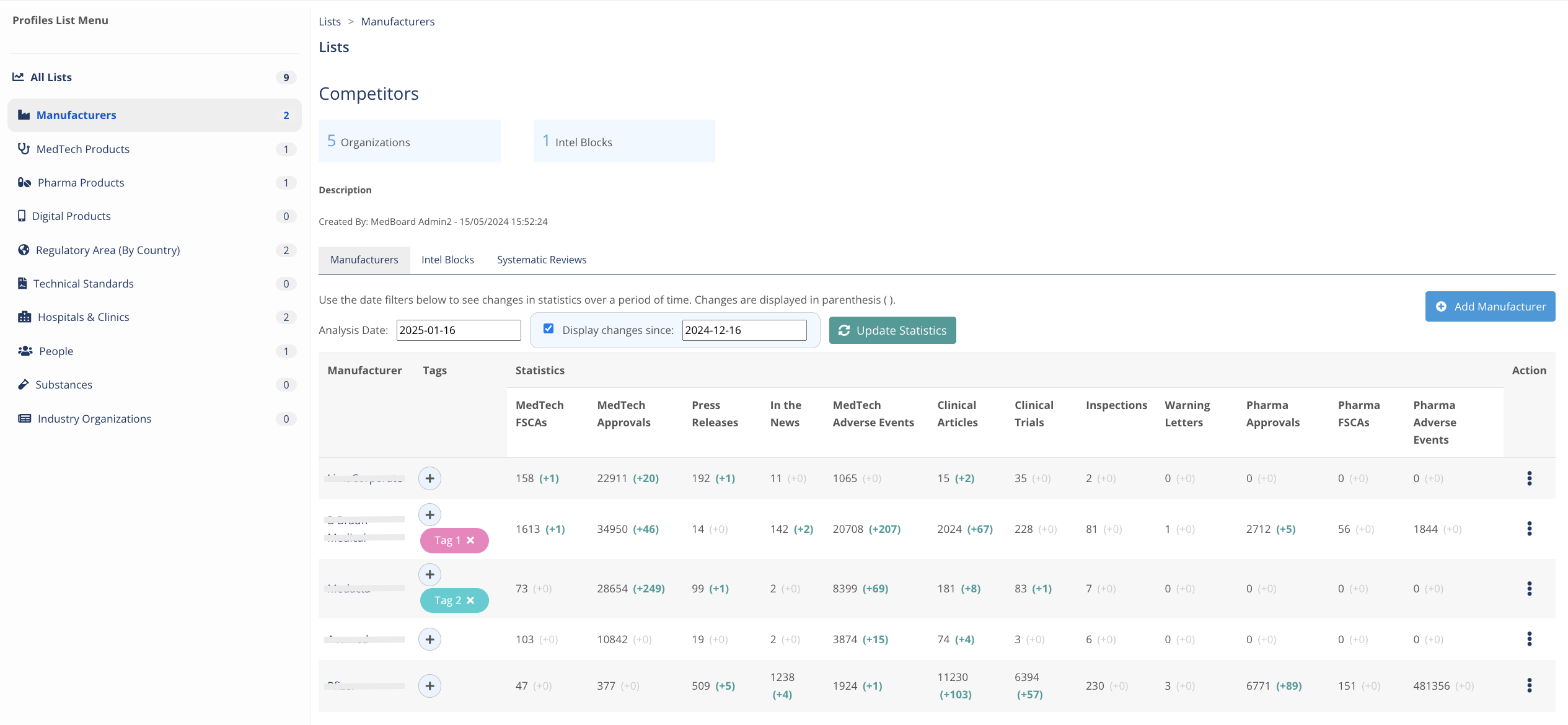
Manage your market lists and actors
Create custom lists with Manufacturers, Products, Substances, Hospitals, Researchers, KOLs, and manny more types from the 10M+ MedBoard Profiles vast collection to analyse changes in statistics/analytics by custom date periods, add intelligence, tags, notes, reviews, and much more in only one place.
Use it as SOTA, CRM or many more uses.
Manage tasks and work implementation
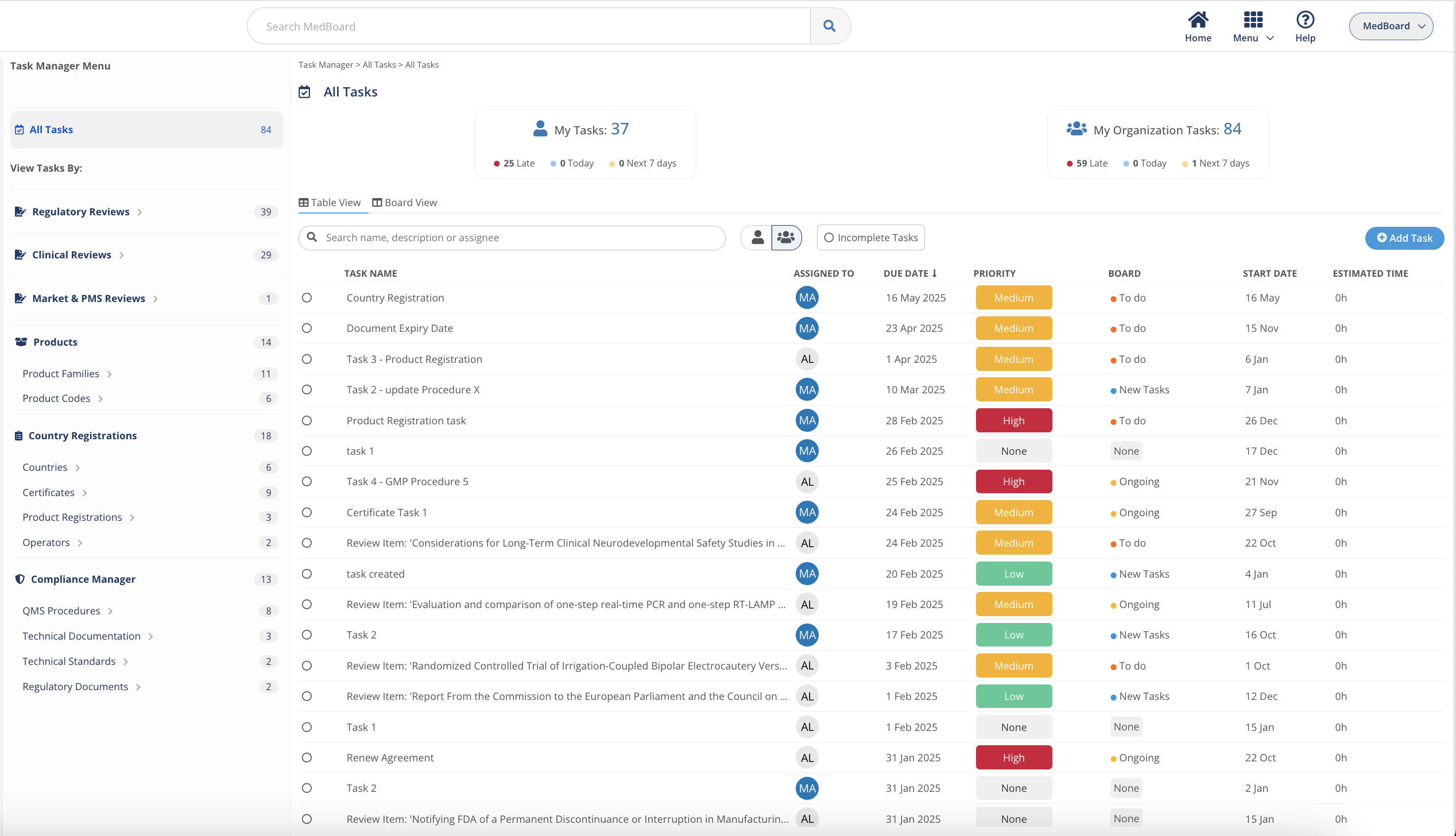
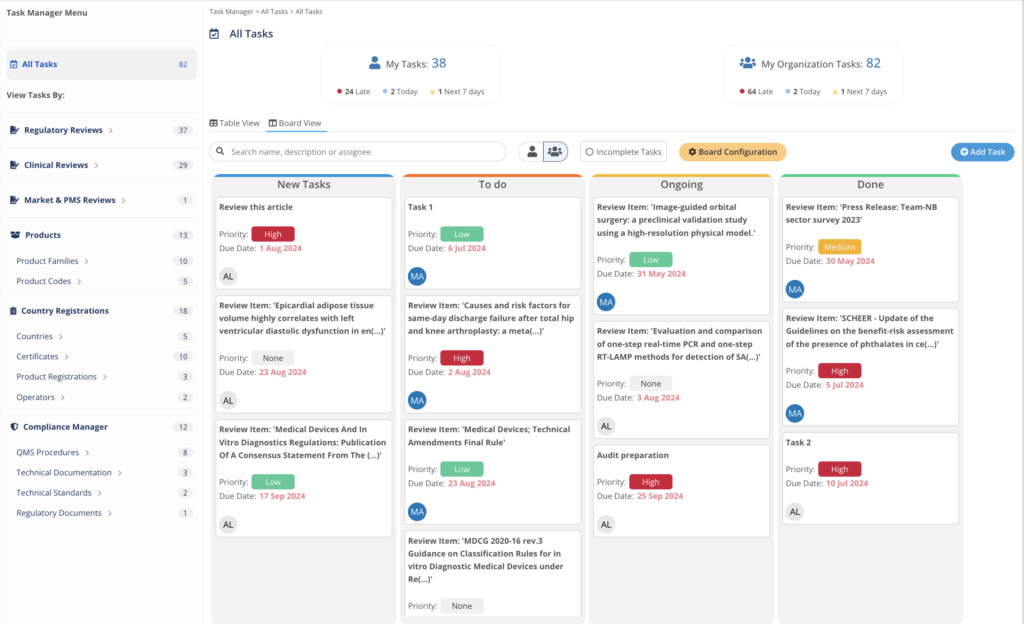
Task Manager integrates with each regulatory MedBoard solution for actions and implementation of tasks. Keep control on deliverables and
- Create Tasks, Actions linked to Regulatory Updates
- Deadlines and Responsible Person
- Email Notifications and Alerts
- Dashboard to report progress and risks
Actions, implementation, and planning managed easily from one single place.
Go Beyond: MedBoard platform offers much more
Discover all our connected solutions
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your evidence transformation journey



