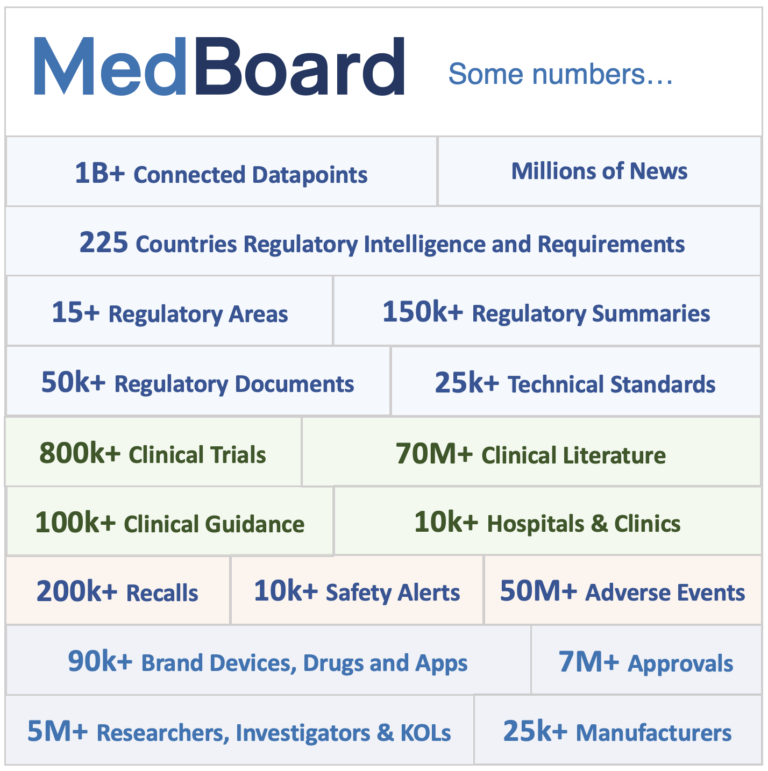
Global Data: Our Data Platform and Knowledge
We transform data and information into knowledge that you can use.
Access information like never before, from the big picture to extreme granularity, to be continuously connected to the latest.
News, Databases, Analytics, Profiles, Intelligence, Resources & Tools
MedBoard is huge, and our Menu provides access to dedicated Regulatory, Clinical, Market, PMS, Standards areas in 225+ Countries, with:
News: Real-Time News from millions of sources such as Authorities, Manufacturers, Clinical Sources, Experts, Hospitals, and any trusted relevant medical stakeholder or source.
Databases & Analytics: 50+ Individual Real-time Trusted Regulatory, PMS, Clinical & Market Databases, with Analytics & Insights. This includes Trials, Recalls, Literature, Regulatory News, Products updates, and much more.
MedBoard Profiles: 10+ Millions of Profiles with integrated data and updates: Countries, Products, Manufacturers, Hospitals, Standards, KOLs, Medical Conditions, Medical Terms, Regulatory Requirements, and many more types.
Intelligence: Databases, Insights & Content, plus curated human intelligence such as 150k+ Country Regulatory Summaries.
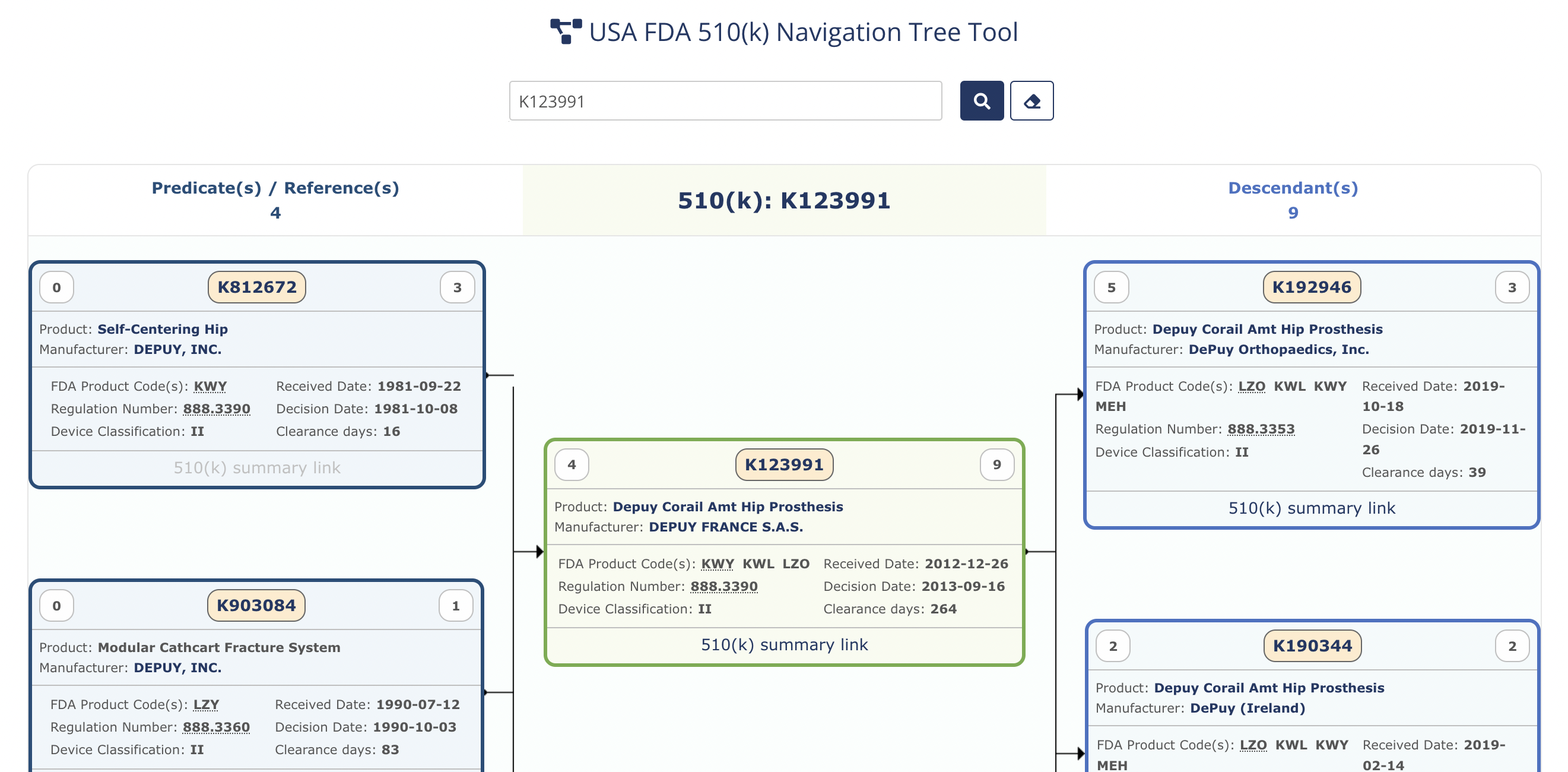
Resources & Tools: Tools, Document Viewers, Translations, Periodic reports, flowcharts, 510(k) Navigator, Dictionaries, Trackers, etc.
MedBoard Search
Search the world’s MedTech, Pharma & Science information with MedBoard, it delivers up to date Regulatory, Clinical, Standards, Clinical, Market and PMS information in more than 225 Countries.
Our Information Portal & Individual databases are curated with only trusted sources and covers more than 1 Billion data points. News, Databases, MedBoard Profiles, Webpages, Documents, Content, Media and much more in just one click.
Regulatory
Access to up to date Regulatory Intelligence, data, tools and News in more than 225 Countries, organized, curated and cleaned by MedBoard.
With a coverage of 15+ regulatory areas, including MedTech, Pharma/BioTech and Clinical trials, that includes countries curated summaries for an unparallel research and intelligence.
Clinical
Access to up to date Clinical databases and information, including Clinical Trials & Studies, Literature, Guidelines, Hospitals and Clinics, professionals & KOLs, medical conditions and treatments, clinical news and much more.
Integrated with advanced filters, analytics and integrations with other MedBoard databases, for a faster research and intelligence.
Market & PMS
Access to up to date Market Databases including Approvals & Registrations, Medical Products profiles (Devices, IVDs, Drugs, Substances, Apps), Medical Manufacturers, Professionals and KOLs, market news and much more.
PMS Vigilance Databases include Safety Alerts & Communications, FSCAs & Recalls, Adverse Events and Shortages. Integrated with advanced filters, analytics and integrations with other MedBoard databases.
Latest News
MedBoard powerful up-to-date platform provides news every day in near real-time, instant to you. You can access to them through the NewsFeed, MedBoard Search, Individual Databases or Profiles.

Regulatory News
225+ Countries, Multiple Authorities in 15+ Regulatory Areas, Technical Standards and much more.

Clinical News
Millions of trusted sources, including hospitals and trials, latest conditions updates and much more.

Market News
Thousands of manufacturers news, press releases, events and much more.
Analytics & Insights: Visualize Data Faster & on the Go
Analytics Studio integration with databases: Search, Filter and Visualize data without the need to use endless hours exporting, copying and pasting into columns in excel and generating graphs. With a query and simple click get access to all information and graphs instantly:
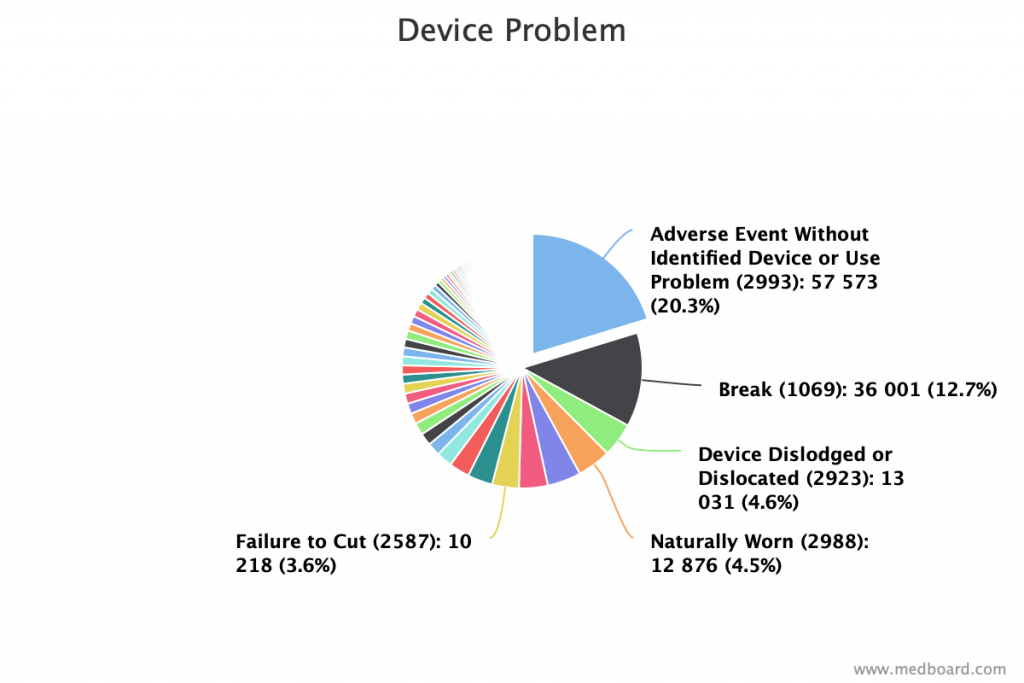
Understand device’s top problems within your area of interest or device types using the analytics.
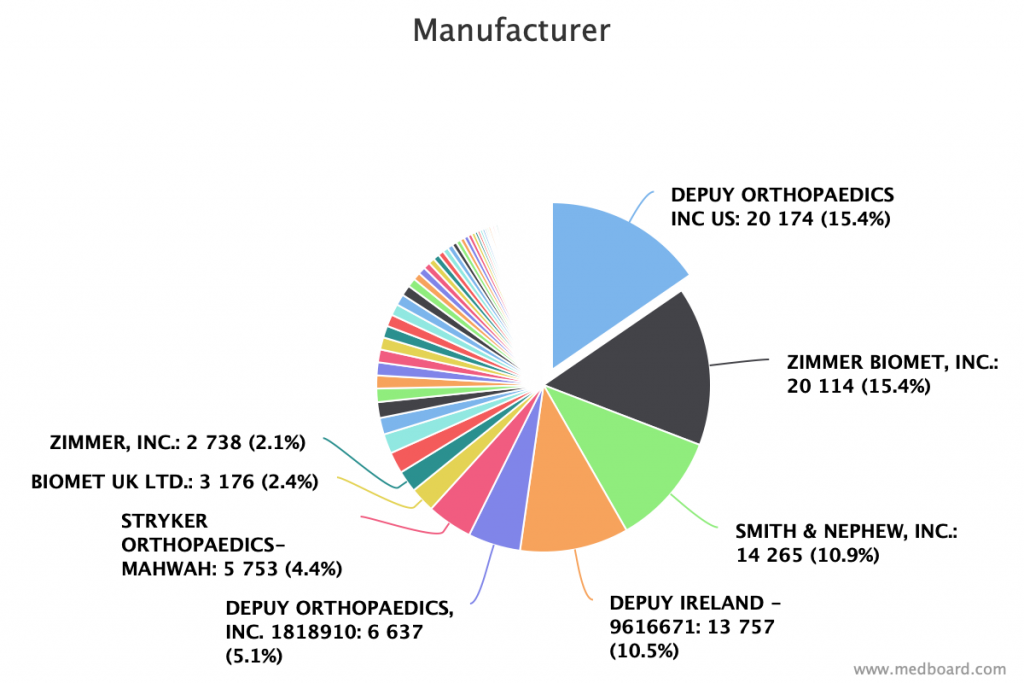
Find which manufacturers have more trials, recalls, literature and many more with just one click.
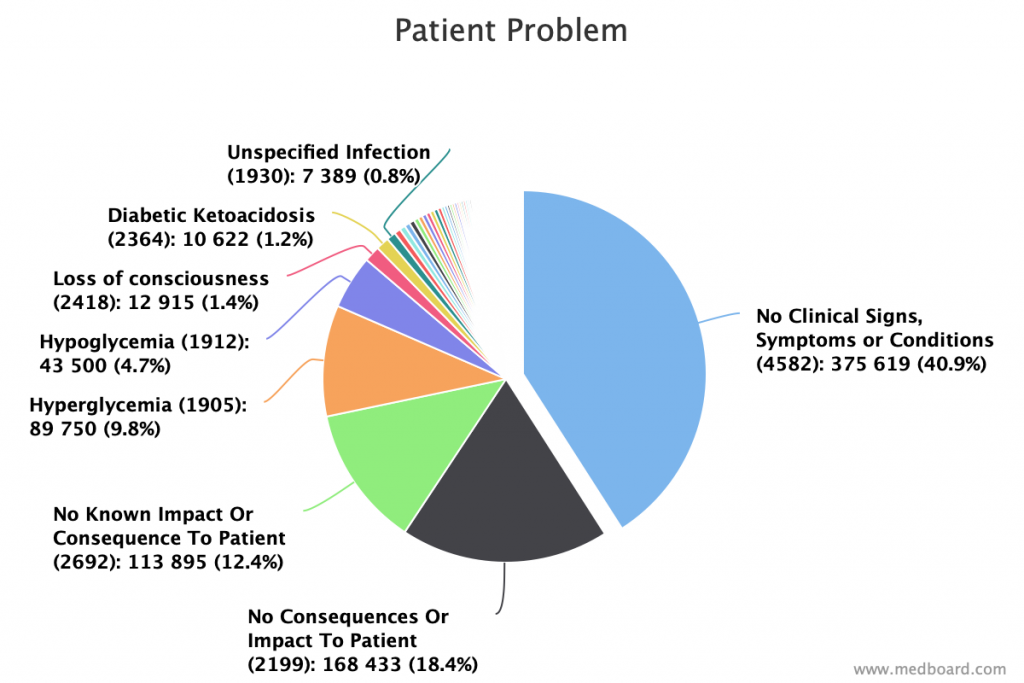
Use databases analytics as patient problems as an input to your organization and processes
Resources & Tools: Understand Data, Connections, Trends & Insights faster
Content is complemented with unique proprietary resources and tools for professionals, in Regulatory, Clinical and Market. Such as: ancillary resources such as reports, insights, rankings, predictions, trends and many more:
• AI Data Extractors
• Daily, Weekly, Monthly and Yearly Reports delivered continuously
Medboard Profiles: Find Countries, Manufacturers & Products on the Market and get their latest related information
Millions of profiles with up-to-date integrated information and data, instant to you. A centralized location where to access instantly to all the news, safety information, analytics, clinical developments and many more related to a profile, where this has been published by public and trusted sources (e.g. authorities or governments). Profiles types include Medical Products, Manufacturers, Medical Conditions and Treatments, Technical Standards, Hospitals, Countries, Authorities, and many more!
” We are enjoying using MedBoard very much. The combination of scientific, regulatory and market information in one well organised place is very appealing to us ”
Go Beyond: MedBoard platform offers even more
Discover all our connected solutions
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your evidence transformation journey




