
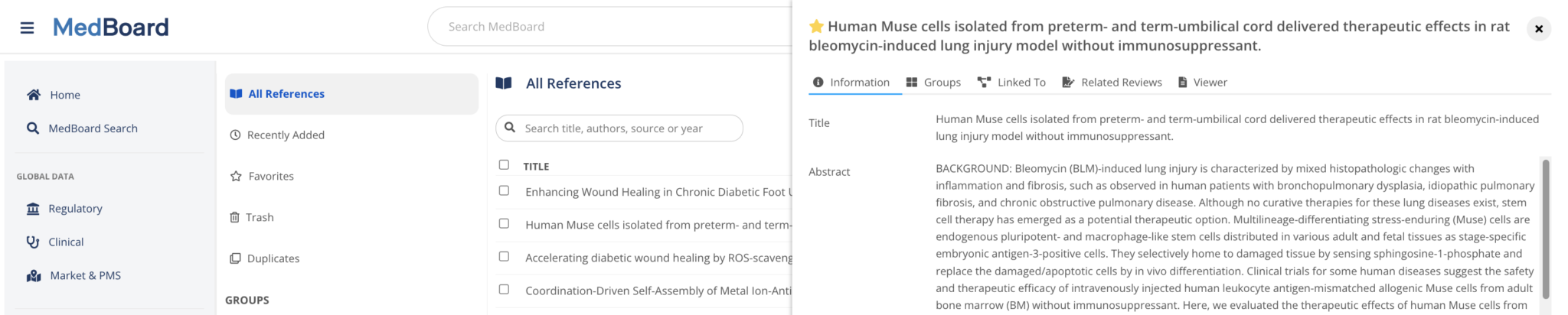
Reference Manager
A customizable and integrated repository to manage and organize your References and PDFs.

Make the Most out of MedBoard
Discover our Software Products for Clinical Needs and Professionals:
![]()
Access to up to date Clinical databases and information, including Clinical Trials & Studies, Literature, Guidelines, Hospitals and Clinics, professionals & KOLs, medical conditions and treatments, clinical news and much more.
Integrated with advanced filters, analytics and integrations with other MedBoard databases, for a faster research and intelligence.
![]()
Review systematically Literature and accelerate your systematic review workflow from search to reporting. Whether you import automatically results or import results from your own databases, MedBoard software and AI-features will simplify and customize to your needs the process, search, review and reporting.
Transfer your Reference from your Literature Systematic Reviews for seamless integration and traceability of Historical Reviews.
![]()
The Task Manager integrates with MedBoard data and modules to easily action, track, and complete efficiently any task and project by teams and professionals. Customize your workflow, and view your projects as a Task List or Board View. Easy to use, with intuitive UI to help you get started fast.
Create Tasks & Action, and connect them to your Evidence to implement any required action.
![]()
A powerful ready-to-use Products Information Management to organize, manage and track information about your products, product codes, SKUs, and its information, including Unique Identifiers (e.g. UDI), all integrated together with Regulatory Intelligence and MedBoard Search.
Link your Evidence to your Products Codes and Families.
![]()
Create custom lists with Manufacturers, Products, Substances, Hospitals, Researchers, KOLs, and manny more types from the 10M+ MedBoard Profiles vast collection to analyse changes in statistics/analytics by custom date periods, add intelligence, tags, notes, reviews, and much more in only one place.
All modules integrate seamlessly with each other,
to keep all connected, in one place.
What do our Customers say about us
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your organization and teams
