- November 27, 2023
- MedBoard
- 0
MedBoard: Introducing End-to-End Regulatory Intelligence
For MedTech, Pharma, BioTech and Digital Health:
A new multi-solution approach, seamlessly integrated, transforming digitally processes and automation, enhancing productivity, and reducing costs and resources.
At MedBoard, we have completed the first series of individual powerful modules, that together, form the most powerful End-to-End Regulatory Intelligence for stakeholders in the industry. This is the outcome of a huge effort by the MedBoard team, combining data science, software engineering and artificial intelligence, aligning with your vision to make medical information accessible and organized, and to enhance productivity of professionals and teams, and reducing costs through innovation.
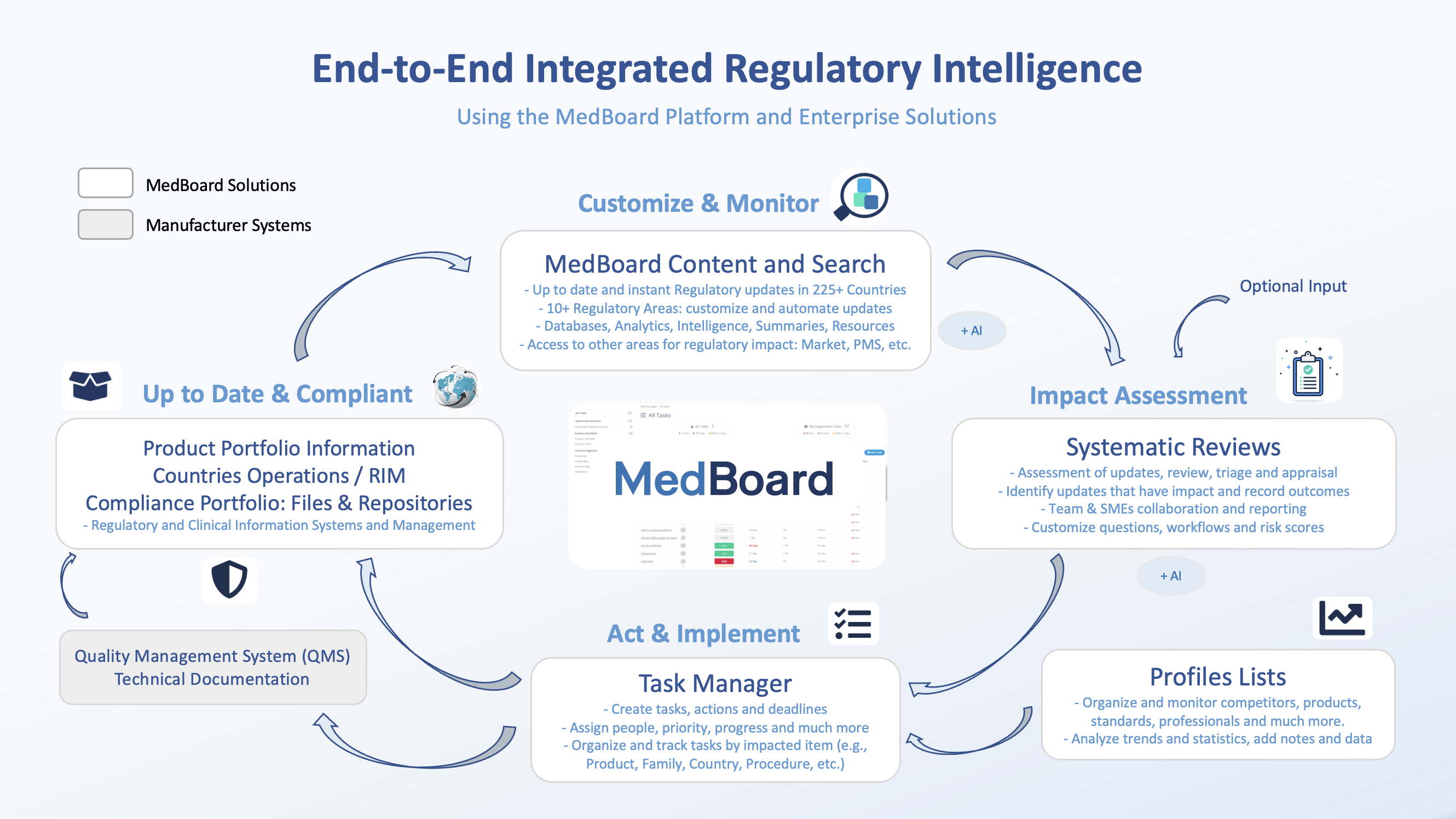
The continuous digital process built with the following modules:
- MedBoard Data Platform: AI Powered Research and Content Access to navigate the complexity of regulations and keep up to date with the continuous changes in many jurisdictions and countries. At MedBoard, we cover 225+ countries and 10+ regulated areas (MedTech, Pharma, Digital Health, Environmental, Packaging, Materials, etc.), including our own synthetic regulatory intelligence summaries by country. Learn more.
- Systematic Reviews: customize the countries and regulatory areas that your organization needs covering, define your process and workflows step by step, your search protocol, appraisal criteria, and systematic review process. Collaborate and directly create tasks to implement actions linked to products, product families, countries, operators, QMS/GMP procedures and much more. Users are supported by AI in this module, extracting and analyzing the document key information and allowing to them review information much faster and integrated. Learn more.
- Profiles Lists: create lists of authorities, manufacturers, products, professionals, that are relevant to the organization, projects, or products. Monitor updates and add your intelligence and information.
- Task Manager: a project management solution fully integrated vertically with other modules. A whole ecosystem of data and processes would not be fully connected if actions and implementations are not linked to ensure that after review and assessment, actions have been assigned, taken, and completed. The Task Manager makes Intelligence actionable and connected. Learn More.
- Regulatory and Clinical Information Systems: such as Product Portfolio Information , Countries Registrations / Operations / RIM, and Compliance Portfolio, where to update and maintain information following any tasks and keep the evidence of compliance and operations. Learn more.
What do we solve?
Tedious manual work, endless number of spreadsheets, integrations, forms, forms and many forms, delayed or lost impact assessments, audits preparations in the last minute, difficulty to keep meeting regulatory, clinical and quality requirements, etc. and much more. Our 100% retention says it all!
Ready to Get Started?
Contact us to know more about this new solution and request a free demo today to see how MedBoard can transform your evidence transformation journey
Do you wish to learn more about MedBoard and this topic?
Do you wish to see MedBoard in action for Regulatory Intelligence and how it simplifies all the above in easy steps?
- Request your demo here.
- For customer success stories and testimionials of organizations already implementing digital processes, click here
- Watch this episode of Easy Medical Device podcast to gain also more insights about Regulatory Intelligence and to this blog.
