The Regulatory, Research & Intelligence Platform
for MedTech | Pharma | Digital Health
A new unique and large ecosystem of data, solutions and enterprise products for medical and life sciences organizations.
Your regulatory, clinical, market, research, intelligence and management on one integrated platform
We’re here to simplify your transformation.
One big data platform, and multiple products for all your needs.
100+ Customers rely on us
Trusted by many leading and innovative organizations. For Small and Large Companies.
Information, Data & Knowledge Platform
MedBoard Platform
MedBoard data platform is huge, we mean, it is actually very huge! (over 1 Billion connected datapoints), and our platform provides global access to information, data and knowledge in all the following areas: Regulatory, Clinical, Market, PMS, Standards and People. Using only trusted sources, and providing continuous and instant access in real-time.
News, Databases, Intelligence, Profiles, Analytics, Tools, Documents, Webpages, Summaries, Translations and many more resources.
MedBoard Search
Search the world’s medical and life sciences information with MedBoard, it delivers up to date Regulatory, Clinical, Standards, Clinical, Market and PMS information in more than 225 Countries, providing relevant results in just one click.
Advanced Filters, AI Evidence Classification and Categorization. Powerful, Up to Date and Continuously Organized.
Premium Intelligence
MedBoard delivers Regulatory Intelligence, Clinical Intelligence & Market Intelligence across the whole platform. It provides an unparalleled Regulatory Intelligence in 225+ countries and more than 15 Regulatory Areas, including MedTech, Pharma, Clinical Trials, Environmental, Data Privacy, Artificial Intelligence (AI), and many more.
Premium Intelligence includes 150,000+ Country Regulatory and Clinical Summaries, curated by experts and continuously evolving and up to date as new changes take place.
“MedBoard is impressive, nothing compares to it”
Enterprise Products
Regulatory Reviews
Our unique Regulatory Review solution is integrated with 225+ countries news and 15 regulatory areas with the latest news and updates in real time. Customize and systematically review regulatory updates and evaluate their impact and implement actions within MedBoard.
Regulatory Intelligence seamless integrated with continuous reviews of Authorities News, Technical Standards updates, Inspections, Warning Letters and other regulatory databases. Show the evidence that you keep track of every day and impact assessment any time, whether is for internal use, customers, or for Authorities.
Clinical & Literature Reviews
Automated and AI-supported clinical and literature reviews to streamline your systematic review workflow from search to reporting. Whether you import automatically results or import results from your own subscriptions, MedBoard workflow software will simplify and customize to your needs the process, review and reporting.
Equipped with AI analyzers for automated data extraction and many other features such as review plans, appraisal tools, duplicates detection, PRISMA 2020 Flowcharts, Keyword highlighting, annotations and many more. Take your team and organization to the next level in consistency, automation and evidence quality!
Market & PMS Reviews
PMS Adverse Events, Recalls, FSCAs, Safety Alerts, Approvals and Registrations, press releases, media news, and many more databases where to choose to perform Market, Post-Market Surveillance (PMS) and Vigilance monitoring.
Fully customize your market reviews by selecting products, manufacturers and many more filters, and continuously keep your reports and stakeholders up to date.
Task Manager
The Task Manager integrates with MedBoard data and products to easily action, track, and complete efficiently any task and project by organization, team and person. Customize your workflow, and view your projects as a Task List or Board View. Easy to use, with intuitive UI to help you get started fast.
Imagine being able to action and implement tasks effortless, the Task Manager makes it possible, and bring collaboration and transparency to your organization.
Compliance Manager
Organize, manage and track your compliance evidence with the Compliance Manager. This product helps to organize and track your compliance evidence with countries, regulations, guidance, standards, procedures, and much more, with dashboards, compliance status trackers, comments, related reviews, tasks, and much more.
Stay compliant and on top in existing jurisdictions, go to new countries, explore new requirements faster, with ready to use templates and AI support, embedded with the latest news of MedBoard Search and MedBoard Intelligence.
Lists
Create custom lists with profiles from the 10M+ MedBoard Profiles vast collection to analyse changes in statistics/analytics by custom date periods, add intelligence, tags, notes, reviews, and much more in only one place. Aggregate in each list the profiles that you need for the project or goal. We have lists for Manufacturers, Products, Authorities, Standards, Healthcare Providers and KOLs.
This is widely used for Research, Intelligence, Due Diligence, SOTA and Clinical Evaluation Reports. Key and essential to understand changes and trends from manufacturers and products and the evolving landscape.
Products Management
A powerful ready-to-use Products Information Management to organize, manage and track information about your products and its information, including UDI, all integrated together with Regulatory Intelligence and MedBoard Search. This module integrates also with other modules such as Country Registrations, and have extensive data features and functions.
Ideal for Manufacturers, Authorised Representatives, Providers, Distributors and other Economic Operators.
Country Registrations RIMS
A powerful ready-to-use Country Registrations RIMS to organize, manage, and track information about your countries registrations, certificates, licenses and economic operators, all integrated together with Regulatory Intelligence, Regulatory Reviews, Task Manager and MedBoard Search.
This Regulatory Information Management System (RIMS) module includes as well many features such as notifications, impact assessments, security controls, document control, notes, priorities. Ideal for Manufacturers, Authorised Representatives, Providers, Distributors and other Economic Operators.
Increase productivity, collaboration, transparency and communication across your organization
For multiple Uses and many Processes:
Seamless integration between enterprise products and platform
Flexible approach to build processes and workflows, easy to implement
Processes within your QMS or QxPs? No problem! we can also support with ready-to-use Software Validations, training, and work instructions.
Testimonials & Customers Stories
“MedBoard is simply amazing and saves me valuable time and effort conducting research and staying up-to-date on changes around the globe. Absolutely the best value for medical device data !!! “
Regulatory Affairs Director
“Having access to a digital platform that acts as a single repository for Regulatory Intelligence, Market Intelligence and Clinical Evidence allows us to automate the laborious tasks and focus on the more technically demanding process. MedBoard is simply, practical tool that offers real value to the organization.”
VP Clinical Affairs & Marketing
“It is our main source for MDR compliant scientific literature searches and post market surveillance. It has been instrumental to optimising these processes by centralised, automated searches, precision of keyword sub-searches, traceable screening and appraisal and useful export functions. We would highly recommended to the medical device industry!
Clinical Evaluator & Project Manager
” WOW!! I am just impressed “
Regulatory Affairs Director
” We are enjoying using MedBoard very much. The combination of scientific, regulatory and market information in one well organised place is very appealing to us. It has significantly reduced the amount of time and effort we spend in many areas. It has been especially helpful for our various regulatory document needs, including following specific regulatory bodies and getting update notifications on regulations. The Literature Search monitoring and traceability has been very useful, and the recent upgrades will make our future use significantly easier.”
Senior Research Officer
Why MedBoard?
The Growing and Long-Term problem that MedBoard Solves

1. An extraordinary increase in flow and speed of news, articles, information and data shared from key sources (e.g. authorities, clinical, manufacturers) that require planning, collecting and reviewing. In addition, there is a growing challenge of ensuring trusted sources and information.

2. A significant increase in demand and organization for data, information, and evidence from key stakeholders (e.g. authorities, payers) and internal processes, and new and increasing larger and more complex requirements for Documentation, Reports, PMS, Clinical Literature review, and data analysis.
Existing solutions are expensive, tedious, cumbersome, and most, still, manual
To start with, none has this amount of data organized, searchable and up to date as MedBoard. Other solutions or alternatives are either manual processes, expensive outsourcing, or repeated searches with no consistency through multiple sites and databases, looking into unstructured and unorganized datasets and manually importing the data.
The MedBoard Approach
The most powerful all-in-one platform while decreasing costs dramatically
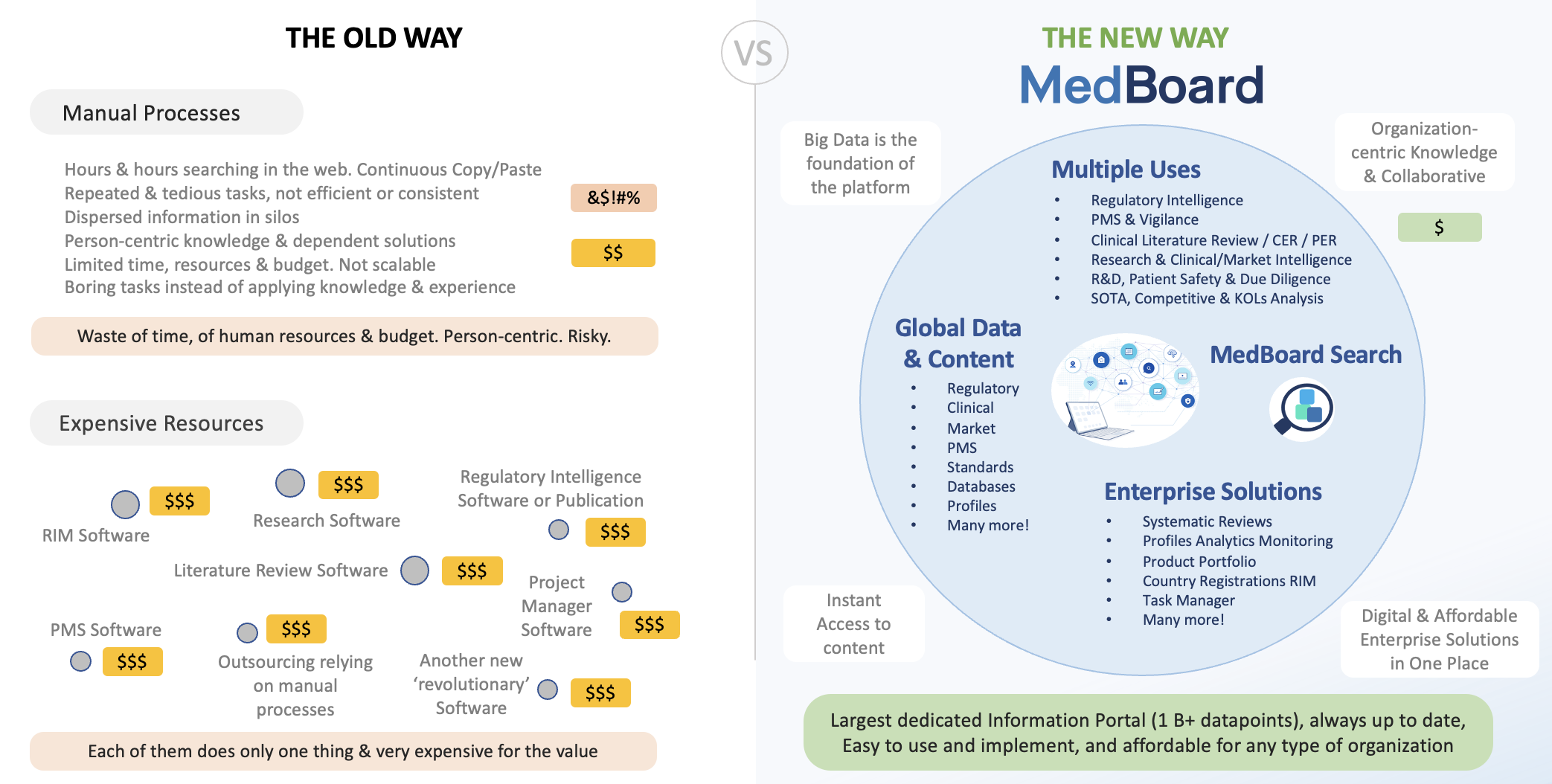
Why Organizations choose MedBoard
MedBoard unique solution is built with the most advanced research features and techniques and integrated into databases and thousands of sources (including importing options), providing seamless integration with trusted data, and easy to use and state of the art tools.

Powerful Search &
Information Access
MedBoard Search, classifications, filters, search operators, and a large number of advanced databases, tools & built in knowledge

Time & Costs Big Savings
Reduce extraordinary amount of work (mostly manual), labour, repetitive tasks and processes with automation, digitization and customization.

Easy to Use & Friendly UI
Customers report 'Easy to Use' and friendly UI as one of the top reasons to use MedBoard, we are transforming complex processes into easy steps.

Multiple Enterprise Solutions
MedBoard is a multi-use platform, providing many solutions in just one place, in addition to incredible access to information and data.

Enterprise Ready
Security, Compliance and Privacy built in features, including SSO are available to our customers. Our platform is also easy to scale for larger customers.

Customization & Teams
Our platform allows teams to fully customize their experience (e.g. review process) and to connect with colleagues and previous work.
Changing Professionals Lives & EMPOWERING them

How many medical professionals with many years of experience, degrees, masters, Phds, are manually searching and doing repetitive tasks, when performing reviews or reporting, or even checking the latest updates. This is mostly due to manual and tedious work (searches, monitoring, reviews and reporting). This is even getting worse and worse over the last years with the unprecedented increase of amount of data shared by Key stakeholders.
And Most Important Question: How are these affecting the quality of life of these professionals? “why high-level professionals should spend hours and hours searching in many locations, or copying and pasting, or other tedious task, instead of applying their knowledge and experience?” We want to change this.
More Accessible & Affordable medical industry information and data, for ALL

We think of the big picture, more accessible information for ALL stakeholders will increase patient safety, innovation, faster execution and quality of life of professionals and patients.
We think of MedBoard as a MUST TO HAVE for any organization working in the life sciences sector. Every organization, regardless their size, should access to and afford such a resource. Pricing to accessible quality databases that are critical for patient safety and medical professionals should never be a barrier. MedBoard is also cloud based and ready-to-use solution.
TRUSTED by leading and world’s best life science organizations of every size. Thousands of users.

Information and data that you can trust. Built by Medical Professionals that already been working in the industry for many years, and Powered by Data Science, engineering and AI. We understand the industry, we understand your challenges and we have great passion for innovation.
A large number of Manufacturers (Medical Devices, In Vitro Diagnostics, Biotech, Pharmaceuticals), Consulting Companies, Providers, Research Organizations and even Governments and Agencies trust and already use MedBoard. The profile of our customers are agile, think-forward, proactive organizations with great understanding of the challenges and the need for adoption of digital solutions.
Trusted, Used and Evaluated by top experts in the industry
Our software has been evaluated by a great number of experts in the industry, which continue to collaborate or work with us. In addition, more formal external evaluations have been taking place and for example, we are proud to have obtained a Digilab Certificate “Certificate for Regulatory Software” issued by the German Company Metecon .
Ready to Get Started?
Request a free demo today to see how MedBoard can transform your evidence, compliance & intelligence transformation journey